A protein can bind two ligands (X and Y) at different sites, thus their binding is not mutually exclusive; the reaction scheme is as follows:
A series of experiments has been carried out in which binding of ligand X has been followed at several (fixed) concentrations of Y: 0, 0.1, 0.3, 1, 3 and 10 mM.
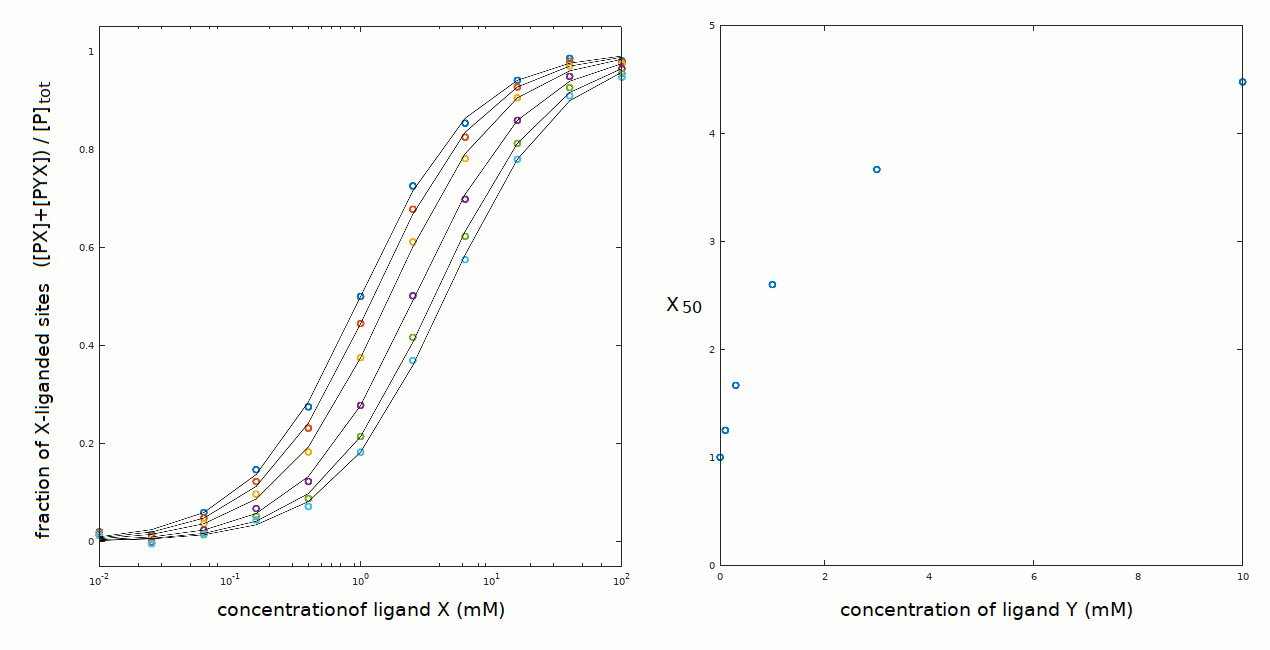
The protein concentration was 0.05 mM, and the protein is pure and monomeric in solution. A common way of representing 2-ligands experiments is to plot the C50 for the ligand whose concentration is varied versus the concentration of the ligand whose concentration is invariant; in this case the X50 as a function of [Y] (left panel). In the present case this plot is a hyperbole.
To proceed with data analysis you need to write down an equation that reflects the raction scheme given above and describes the fraction of sites bound with ligand X as a function of the concentrations of ligands X and Y. For the present tutorial we assume that the signal you use faitfully monitors either [PX] or [PX]/[P]tot. Write down the equation you need and then proceed to next step.