In an experiment aimed at determining the equilibrium constant of the reaction P + X <==> PX, the following data set has been collected:
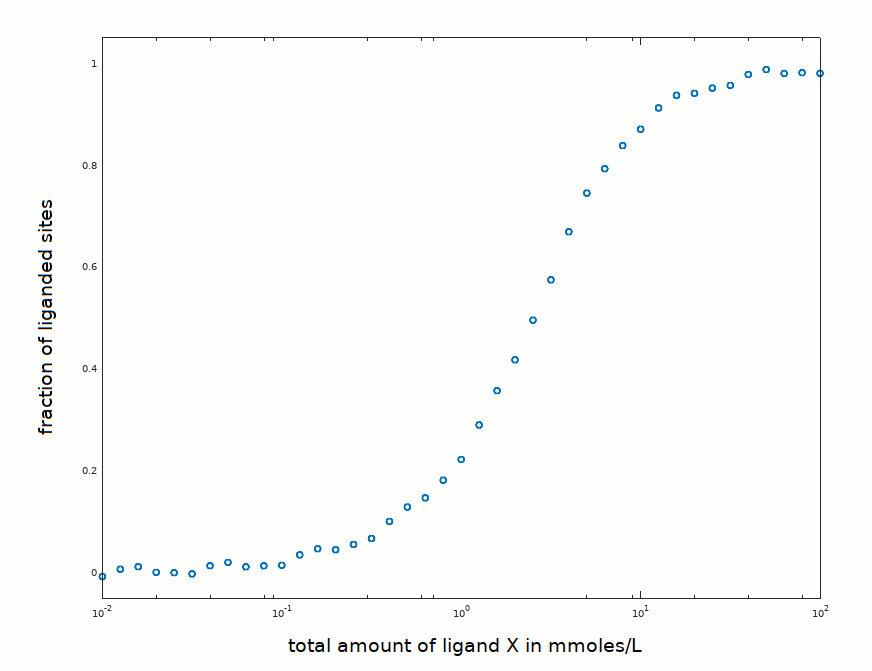
The protein concentration was 3 mM, of the same order of magnitude as the ligand concentration required to observe binding. Structural information is available and shows that the protein is a monomer in solution; moreover it is a pure preparation, and not a mixture of isoforms. It is thus expected to obey the law Y = [X] / (K +[X]).
How would you proceed with data analysis (click to select)?
1) Fit eq. Y = [X]/(K+[X]) to the experimental data in order to find the value of K that minimizes the sum of the squared residuals.
2) Linearize the data using Hill's equation log(Y/(1-Y)) = n log([X]) - n log(K) then draw the best straight line through the data points in order to find K and n.