answer 5-5: stoichiometric titration of the protein with the ligand
We demonstrated that the free ligand concentration required to saturate half the available binding sites (C50) equals the Kd (in more complex reaction schemes C50 may a function of Kd and other parameters). However, in the case of a very high affinity ligand C50 ≅ zero. Thus in this case we cannot determine a Kd. The data set used in this example was calculated for Kd = 0.1 mM, i.e. Kd = [P]tot / 30, but in a real data set, which contains experimental errors, the conclusion we can draw is Kd << [P]tot.
The presence of experimental errors causes the second degree equation we used in tutorial #4 to be inadequate in the present case, and Kd is undetermined except for the above reported conclusion that it is much lower than [P]tot.
This experiment, in spite of the fact that sets an upper limit to Kd, contains very relevant information on the protein. Indeed it gives the absolute concentration [PX]=[X]tot, and hence the stoichiometry of the reaction. If we approximate our decription to two straight lines, we read at their intercept the number of ligand binding sites presnet in solution, as shown in the following figure.
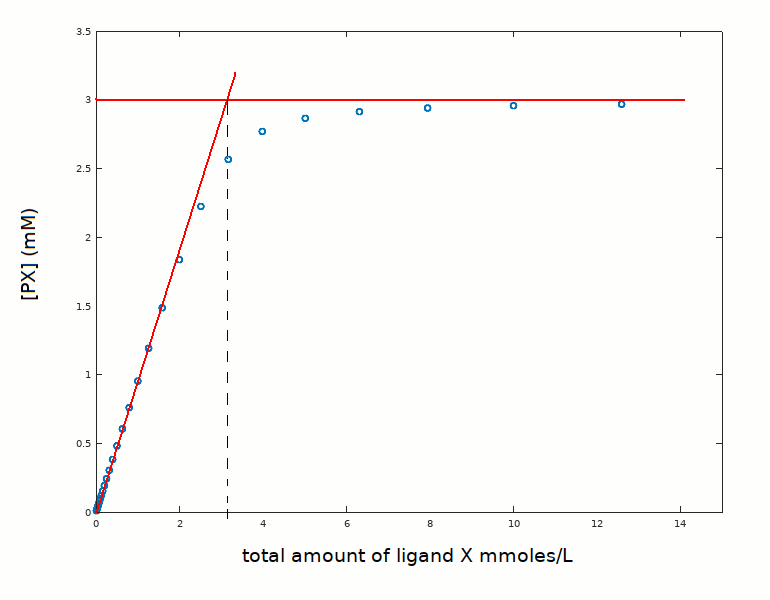
The present example was simulated under the assumption of the protein to be a single-site monomer at 3 mM concentration. However, in real cases we may have an estimate of the MW of the protein and know that it is a monomer at 3 mM concentration, but we may not know how many ligand binding sites the monomer contains. Calmodulin, for example, is a monomer but has four calcium binding sites. An experiment carried out under conditions of very high ligand affinity is ideal to measure the number of binding sites per monomer.
It is important to state once more that high and very high affinity describe a relationship between [P]tot and Kd rather than some predefined absolute value of Kd and it is possible that, by changing the experimental conditions and detection method the same protein-ligand couple switches from a condition of very high affinity (Kd << [P]tot) to one of high affinity (Kd ≅ [P]tot) or even to Kd >> [P]tot. An interesting example is provided by the reaction of hemoproteins with oxygen. For most hemoglobins and myoglobins the typical C50 of O2 is in the tens of micromolar range (imagine here an experiment carried out using O2 in solution, in the absence of a free gas phase). Oxygen binding to hemoproteins is conveniently detected by absorbance spectroscopy and the proteins have extinction coefficients that range from over 105 M-1cm-1 in the Soret wavelength range to 104 M-1cm-1 in the visible range and less than 103 M-1cm-1 in the far red region of the spectrum. Thus, by appropriately selecting the spectral region to be used one can detect oxygen binding to hemoglobin at concentrations ranging from micromolar to millimolar effectivaely spanning from [P]tot < Kd to [P]tot > Kd.
There are cases in which there is no way to reach the condition Kd >> [P]tot; in these cases a measurement of Kd can only be achieved by means of replacement reactions (see tutorial #7) or in the presence of heterotropic effectors, if these exist (see tutorial #8).
This tutorial is complete. Back to table of content